SciScout, headquartered within minutes of Research Triangle Park, NC, provides life sciences consulting
services to companies within the pharmaceutical, chemical and related industries.
Toxicology Assessment Services
SciScout is contracted by pharmaceutical, biotechnology and related companies to produce study reports assessing possible toxicity and human health risk of chemical contaminants and other impurities associated with their products. Our clients submit our detailed study reports along with other documents as part of their regulatory submission process.
We develop our toxicology assessment reports from an extensive search and review of the scientific literature, accessible databases and use of analytical data for extractables, leachables, particles or other product-associated contaminants provided by our clients. We apply structure-activity relationships (SAR) and other comparative methods to assist in assessments when available toxicology literature is limited for some compounds. Reference Doses (RfDs) and Acceptable Daily Intakes (ADIs) of compounds are computed from No Observed Adverse Effect Levels (NOAELs). Patient exposure length, administration route and patient population are all considered when assessing possible toxicity and human health risk.
Example Toxicity Assessment Report (Target Leachables)
A SciScout literature-based toxicological assessment of eight target leachables associated with a biomanufactured drug product. This assessment was conducted to assess toxicological implications and to determine safe levels for the compounds.
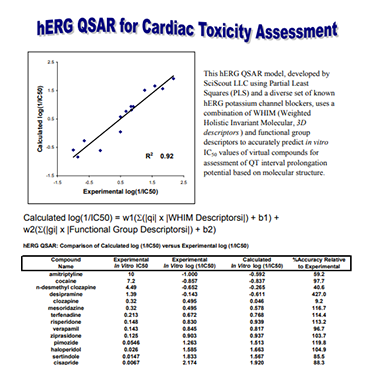
hERG QSAR Example
This hERG QSAR model, developed by SciScout using Partial Least Squares (PLS) and a diverse set of known hERG potassium channel blockers, uses a combination of WHIM (Weighted Holistic Invariant Molecular, 3D descriptors) and functional group descriptors to accurately predict in vitro IC50 values of virtual compounds for assessment of QT interval prolongation potential based on molecular structure. This QSAR model of hERG binding is defined by:
Calculated log(1/IC50) = w1(S(|qi| x |WHIM Descriptorsi|) + b1) + w2(S(|gi| x |Functional Group Descriptorsi|) + b2) QSAR modeling services are available on a limited basis. Contact us to inquire about this service.
Safety Assesment Of Inhalable Particles
We group inhalable particulate contaminants into categories based on size and chemical composition when assessing their human health risk. "Worst case” masses of identified particles are computed across grouped particle size ranges and for all identified chemical compositions, taking into account various dose-related factors. The mathematical relationship between particle size and mass as defined by m = ρ4π(d/2)3/3), where m is mass, ρ is density and d is diameter, serves as the basis for the computation of mass.
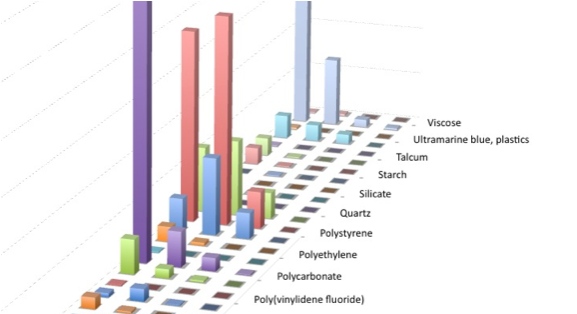
Relative number count, size distribution and chemical composition of 2–100 µm diameter particulate contaminants extracted from a metered-dose inhaler.
RAMAN data provided by our client were used to generate this partial visualization.